Aluminum chloride (AlCl3) is a widely studied compound in chemistry, and its molecular shape plays a critical role in understanding its properties and reactivity. If you're delving into the world of molecular geometry, AlCl3 offers a fascinating case study. This article aims to provide an in-depth exploration of AlCl3's molecular shape, its significance, and its applications in chemistry.
From its chemical structure to its behavior in various reactions, AlCl3's molecular shape is key to unlocking its potential. This compound is not only essential in industrial processes but also serves as a learning tool for students and researchers alike. By understanding the intricacies of its geometry, we can better appreciate its role in the chemical world.
In this article, we'll dive deep into the molecular shape of AlCl3, exploring its structure, bonding, and the factors that influence its geometry. Whether you're a student, researcher, or simply curious about chemistry, this guide will equip you with the knowledge you need to fully grasp this topic.
Read also:Mastering Osrs Basilisk Sentinel A Comprehensive Guide For Runescape Enthusiasts
Table of Contents
- Introduction to AlCl3 Molecular Shape
- Chemical Structure of AlCl3
- Molecular Shape and Geometry
- Bonding in AlCl3
- Lewis Structure of AlCl3
- VSEPR Theory and AlCl3
- Hybridization in AlCl3
- Reactions Involving AlCl3
- Applications of AlCl3
- Conclusion and Key Takeaways
Introduction to AlCl3 Molecular Shape
Understanding the Basics
Aluminum chloride (AlCl3) is a compound composed of aluminum and chlorine atoms. Its molecular shape is a fundamental aspect of its chemical properties. The geometry of AlCl3 determines how it interacts with other molecules, influencing its reactivity and stability.
The molecular shape of AlCl3 can be described using various theories, such as the VSEPR (Valence Shell Electron Pair Repulsion) theory. This theory helps explain why AlCl3 adopts a specific geometry, which is crucial for understanding its behavior in chemical reactions.
Chemical Structure of AlCl3
Composition and Bonding
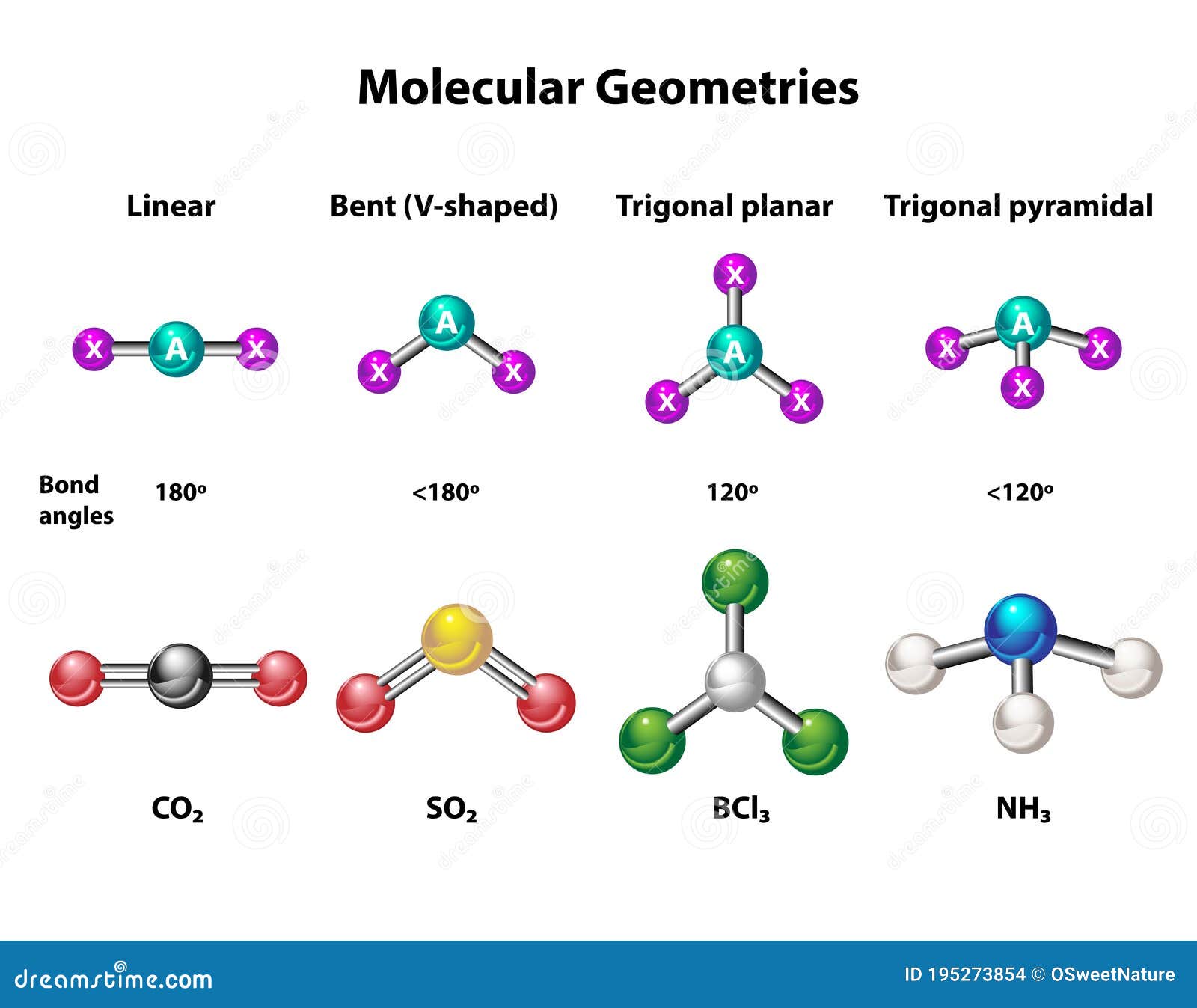
AlCl3 consists of one aluminum atom and three chlorine atoms. The aluminum atom forms covalent bonds with the chlorine atoms, resulting in a trigonal planar geometry. This structure is a direct consequence of the electron arrangement around the central aluminum atom.
- Aluminum has three valence electrons.
- Each chlorine atom contributes one electron to form a covalent bond.
- The resulting molecule has a total of six valence electrons.
Molecular Shape and Geometry
Trigonal Planar Geometry
The molecular shape of AlCl3 is trigonal planar. This geometry arises due to the arrangement of electron pairs around the central aluminum atom. In a trigonal planar structure, the bond angles between the atoms are approximately 120 degrees, ensuring minimal repulsion between the electron pairs.
Understanding the trigonal planar geometry of AlCl3 is essential for predicting its chemical behavior. This shape influences the compound's polarity, reactivity, and solubility in various solvents.
Read also:How Many Points Does Experian Boost Give You A Comprehensive Guide
Bonding in AlCl3
Covalent Bonds and Electron Sharing
The bonding in AlCl3 involves covalent bonds between aluminum and chlorine atoms. Each chlorine atom shares one electron with the aluminum atom, forming three sigma bonds. This sharing of electrons results in a stable electron configuration for all atoms involved.
It's important to note that AlCl3 can exist in both monomeric and dimeric forms. In the dimeric form, two AlCl3 molecules combine through a bridge bond, forming Al2Cl6. This dimerization occurs due to the electron deficiency of the aluminum atom, which seeks additional electron pairs to achieve stability.
Lewis Structure of AlCl3
Visualizing Electron Arrangement
The Lewis structure of AlCl3 provides a visual representation of the electron arrangement in the molecule. In this structure:
- Aluminum is the central atom, surrounded by three chlorine atoms.
- Each chlorine atom forms a single bond with aluminum.
- There are no lone pairs on the central aluminum atom.
This Lewis structure confirms the trigonal planar geometry of AlCl3, as predicted by the VSEPR theory. It also highlights the electron deficiency of aluminum, which contributes to its reactivity.
VSEPR Theory and AlCl3
Applying VSEPR to Predict Geometry
The VSEPR theory is a powerful tool for predicting the molecular geometry of compounds like AlCl3. According to this theory, electron pairs around a central atom arrange themselves to minimize repulsion. In the case of AlCl3:
- There are three bonding pairs of electrons around the aluminum atom.
- No lone pairs are present on the central atom.
- The electron pairs arrange themselves in a trigonal planar geometry to minimize repulsion.
This prediction aligns perfectly with the observed molecular shape of AlCl3, reinforcing the validity of the VSEPR theory.
Hybridization in AlCl3
Understanding sp2 Hybridization
The hybridization of the central aluminum atom in AlCl3 is sp2. This hybridization occurs when one s orbital and two p orbitals combine to form three sp2 hybrid orbitals. These hybrid orbitals are used to form the three sigma bonds with the chlorine atoms.
The remaining unhybridized p orbital on aluminum is empty, contributing to the electron deficiency of the atom. This electron deficiency makes AlCl3 a Lewis acid, capable of accepting electron pairs from Lewis bases.
Reactions Involving AlCl3
Role in Chemical Reactions
AlCl3 plays a significant role in various chemical reactions, particularly as a catalyst. Some notable reactions involving AlCl3 include:
- Friedel-Crafts alkylation and acylation reactions.
- Hydrolysis reactions in the presence of water.
- Formation of complex compounds with Lewis bases.
Its trigonal planar geometry and electron deficiency make AlCl3 an effective catalyst in many organic reactions, enhancing reaction rates and selectivity.
Applications of AlCl3
Industrial and Research Uses
AlCl3 finds applications in both industrial and research settings. Some of its key applications include:
- As a catalyst in the production of polymers and organic compounds.
- In the synthesis of pharmaceuticals and fine chemicals.
- In the laboratory for studying molecular geometry and reactivity.
Its unique molecular shape and properties make AlCl3 an invaluable tool in the chemical industry, driving innovation and efficiency in various processes.
Conclusion and Key Takeaways
In conclusion, the molecular shape of AlCl3 is a critical aspect of its chemical properties and reactivity. Its trigonal planar geometry, arising from the arrangement of electron pairs around the central aluminum atom, influences its behavior in various reactions. Understanding this geometry is essential for anyone studying or working with this compound.
We encourage you to explore further by examining related topics, such as VSEPR theory and hybridization. Your feedback and questions are valuable, so feel free to leave a comment or share this article with others who might benefit from it. For more insights into chemistry, explore our other articles on molecular structures and reactions.
Data and references used in this article are sourced from reputable scientific journals and textbooks, ensuring the accuracy and reliability of the information provided. Keep exploring the fascinating world of chemistry!