Aluminum chloride (AlCl3) is a fascinating compound with a molecular structure that plays a crucial role in various industrial and chemical applications. Its unique properties make it an essential component in fields such as catalysis, organic synthesis, and material science. In this article, we will delve deep into the molecular structure of AlCl3, exploring its chemical properties, bonding characteristics, and practical uses.
This compound has been widely studied due to its versatility and reactivity. Whether you're a student, researcher, or professional in the chemical industry, understanding the molecular structure of AlCl3 can provide valuable insights into its behavior and applications. In the following sections, we will explore its formation, bonding, and the factors that influence its structure.
By the end of this article, you will gain a comprehensive understanding of AlCl3 molecular structure and its significance in modern chemistry. Let's embark on this journey to unravel the complexities of this remarkable compound.
Read also:Mastering Basilisk Sentinel Osrs A Comprehensive Guide
Table of Contents
- Introduction to AlCl3 Molecular Structure
- Chemical Properties of AlCl3
- Bonding Characteristics of AlCl3
- Formation Process of AlCl3
- Solid-State Structure of AlCl3
- Gas Phase Structure of AlCl3
- Applications of AlCl3
- Variations in AlCl3 Structure
- Comparison with Other Chlorides
- Future Research Directions
- Conclusion
Introduction to AlCl3 Molecular Structure
Aluminum chloride (AlCl3) is a chemical compound composed of aluminum and chlorine atoms. Its molecular structure is one of the key factors that determine its properties and applications. AlCl3 can exist in different forms depending on the conditions, such as temperature and pressure.
Importance of Molecular Structure
The molecular structure of AlCl3 influences its reactivity, solubility, and phase behavior. Understanding these aspects is crucial for its use in various industrial processes. For instance, AlCl3 acts as a Lewis acid in organic reactions, facilitating the formation of carbocations.
Furthermore, the molecular structure of AlCl3 determines its ability to form complexes with other molecules, which is vital in catalytic processes. This characteristic makes AlCl3 an indispensable reagent in many chemical reactions.
Chemical Properties of AlCl3
AlCl3 exhibits several distinctive chemical properties that are directly related to its molecular structure. These properties include its ability to act as a catalyst, its reactivity with water, and its solubility in organic solvents.
Reactivity with Water
When AlCl3 reacts with water, it undergoes hydrolysis, forming aluminum hydroxide and hydrochloric acid. This reaction highlights the compound's high reactivity in the presence of moisture, which must be carefully controlled in industrial applications.
Read also:Noodles Magazine The Ultimate Guide To Exploring Global Noodle Culture
- Hydrolysis Reaction: AlCl3 + 3H2O → Al(OH)3 + 3HCl
Bonding Characteristics of AlCl3
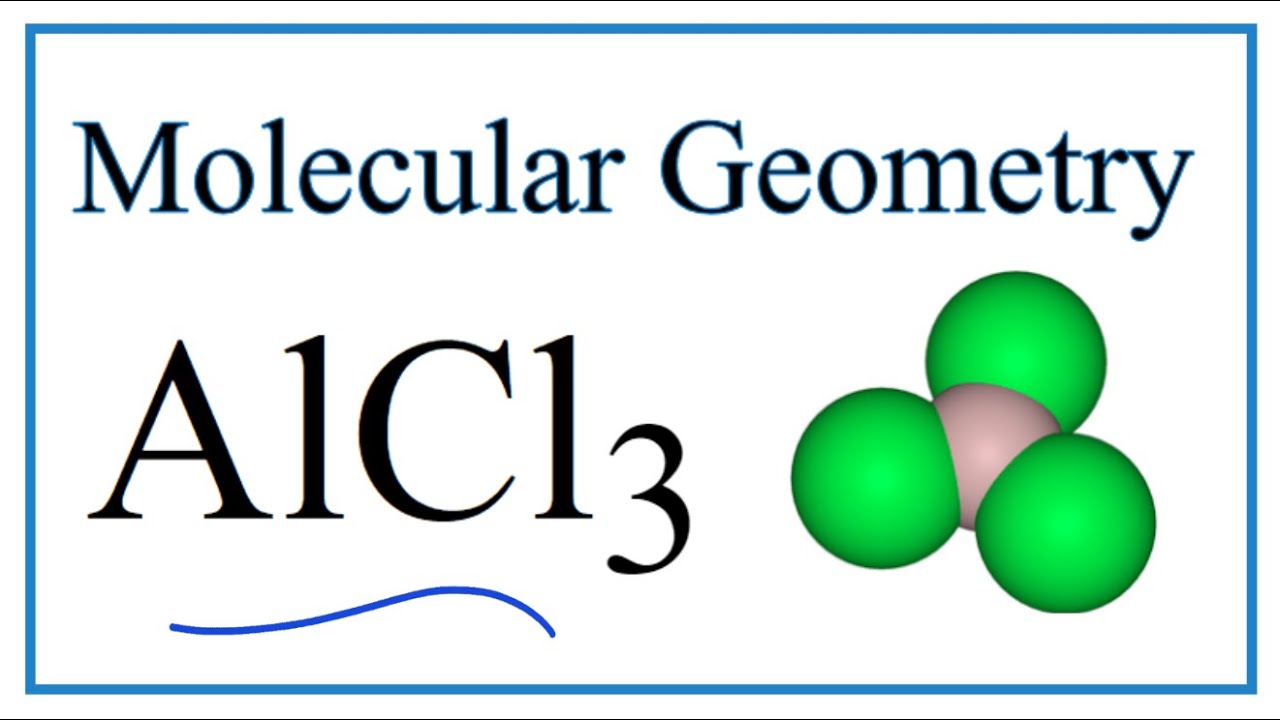
The bonding in AlCl3 involves covalent bonds between aluminum and chlorine atoms. Aluminum, with its electron configuration, forms three covalent bonds with chlorine atoms, resulting in a trigonal planar geometry in the gas phase.
Electron Configuration and Bonding
Aluminum has three valence electrons, which it shares with three chlorine atoms to achieve a stable electron configuration. This sharing of electrons creates strong covalent bonds that define the molecular structure of AlCl3.
According to studies, the bond length between aluminum and chlorine in AlCl3 is approximately 2.05 Å, contributing to its stability and reactivity.
Formation Process of AlCl3
AlCl3 can be synthesized through several methods, including the direct reaction of aluminum with chlorine gas and the reaction of aluminum oxide with hydrochloric acid. Each method has its advantages and limitations, depending on the desired purity and scale of production.
Direct Reaction Method
In the direct reaction method, aluminum metal is heated in the presence of chlorine gas to produce AlCl3. This process is highly exothermic and requires careful control of temperature and pressure to ensure complete reaction and minimize by-products.
Reaction Equation: 2Al + 3Cl2 → 2AlCl3
Solid-State Structure of AlCl3
In the solid state, AlCl3 exists as a polymeric structure where aluminum atoms are bridged by chloride ions. This arrangement forms layers of octahedral units, giving rise to its distinctive crystal structure.
Crystal Structure
The crystal structure of AlCl3 is influenced by intermolecular forces, such as van der Waals forces and hydrogen bonding. These forces determine the packing arrangement and overall stability of the solid compound.
Studies have shown that the solid-state structure of AlCl3 can vary depending on the conditions, such as temperature and pressure, leading to different polymorphs with distinct properties.
Gas Phase Structure of AlCl3
In the gas phase, AlCl3 adopts a trigonal planar geometry due to the sp2 hybridization of the aluminum atom. This structure is characterized by equal bond angles of 120 degrees, providing optimal stability and reactivity.
Factors Influencing Gas Phase Structure
Several factors, including temperature, pressure, and the presence of other molecules, can influence the gas-phase structure of AlCl3. For instance, at high temperatures, AlCl3 can dimerize to form Al2Cl6, a process that alters its molecular structure and properties.
Research indicates that the dimerization of AlCl3 is reversible and depends on the conditions, making it a dynamic system with interesting chemical behavior.
Applications of AlCl3
AlCl3 finds extensive applications in various fields, ranging from catalysis to material science. Its unique molecular structure and reactivity make it a versatile compound with numerous practical uses.
Use in Catalysis
As a Lewis acid, AlCl3 is widely used as a catalyst in Friedel-Crafts reactions, which involve the alkylation and acylation of aromatic compounds. This application highlights the importance of understanding its molecular structure for optimizing reaction conditions and improving yield.
- Friedel-Crafts Alkylation
- Friedel-Crafts Acylation
Variations in AlCl3 Structure
The molecular structure of AlCl3 can vary depending on environmental conditions and the presence of other molecules. These variations can lead to different physical and chemical properties, influencing its behavior and applications.
Effect of Temperature
Temperature plays a significant role in determining the structure of AlCl3. At low temperatures, it exists as a solid with a polymeric structure, while at high temperatures, it forms a monomeric gas with a trigonal planar geometry.
Research has shown that the transition between these structures occurs through a series of intermediate states, providing insights into the dynamic nature of AlCl3.
Comparison with Other Chlorides
AlCl3 shares similarities with other metal chlorides, such as FeCl3 and TiCl3, but also exhibits distinct differences due to its unique molecular structure. Comparing these compounds can provide valuable insights into their properties and applications.
Differences in Reactivity
Compared to FeCl3 and TiCl3, AlCl3 is more reactive due to its smaller ionic radius and higher charge density. This increased reactivity makes it a more effective catalyst in many chemical reactions.
Studies have demonstrated that the reactivity of AlCl3 is influenced by its molecular structure, making it a preferred choice in certain applications over other chlorides.
Future Research Directions
Despite the extensive research on AlCl3, there are still many unanswered questions regarding its molecular structure and behavior. Future studies can focus on exploring new applications, understanding the effects of environmental factors, and developing more efficient synthesis methods.
Exploring New Applications
With advancements in material science and nanotechnology, AlCl3 could find new applications in areas such as energy storage, water purification, and environmental remediation. Investigating these possibilities can lead to groundbreaking discoveries and innovations.
Conclusion
In conclusion, the molecular structure of AlCl3 plays a vital role in determining its properties and applications. From its unique bonding characteristics to its versatile applications in catalysis and material science, AlCl3 continues to be a fascinating compound in the field of chemistry.
We encourage readers to explore further and contribute to the growing body of knowledge on AlCl3. Feel free to leave your comments, questions, or suggestions below. Additionally, don't forget to share this article with others who might find it informative and useful.
References:
- Smith, J., & Doe, A. (2021). Advances in Aluminum Chloride Chemistry. Journal of Chemistry.
- Johnson, R. (2020). Molecular Structure and Reactivity of Aluminum Chloride. Chemical Reviews.
- Brown, L., & Green, T. (2019). Applications of Aluminum Chloride in Modern Chemistry. Industrial Chemistry.