Aluminum chloride (AlCl3) is a fascinating compound that plays a significant role in chemistry, particularly in understanding its Lewis structure and molecular geometry. Its properties and structure are crucial for various industrial and scientific applications. In this article, we will explore the intricacies of AlCl3, starting from its basic structure to its advanced properties.
When discussing chemical compounds, the Lewis structure and molecular geometry provide essential insights into how atoms bond and arrange themselves in space. For AlCl3, these concepts are fundamental for predicting its reactivity and behavior in different environments.
This article will serve as a detailed guide to understanding the Lewis structure, molecular geometry, and other related properties of AlCl3. Whether you're a student, researcher, or simply curious about chemistry, this comprehensive exploration will provide valuable insights into this compound's structure and applications.
Read also:How Much Is Jb Mauney Worth Exploring The Wealth And Career Of A Bull Riding Champion
Table of Contents
- AlCl3 Lewis Structure
- Molecular Geometry of AlCl3
- Bonding in AlCl3
- Hybridization in AlCl3
- Physical and Chemical Properties
- Applications of AlCl3
- Variations in AlCl3 Structure
- Synthesis of AlCl3
- Safety Considerations
- Conclusion
AlCl3 Lewis Structure
The Lewis structure of AlCl3 provides a visual representation of how electrons are distributed between atoms in the compound. Aluminum (Al) is the central atom, surrounded by three chlorine (Cl) atoms. Each chlorine atom contributes one electron to form a single covalent bond with aluminum, resulting in a total of six valence electrons shared in the structure.
In the Lewis structure, aluminum has an incomplete octet, meaning it does not satisfy the octet rule. This characteristic makes AlCl3 an electron-deficient molecule, which influences its reactivity and ability to form complexes with other molecules.
Steps to Draw the Lewis Structure
- Identify the total number of valence electrons: Aluminum contributes 3 electrons, and each chlorine contributes 7 electrons, totaling 24 valence electrons.
- Place aluminum as the central atom and chlorine atoms around it.
- Form single bonds between aluminum and each chlorine atom.
- Complete the octets of the chlorine atoms by adding lone pairs.
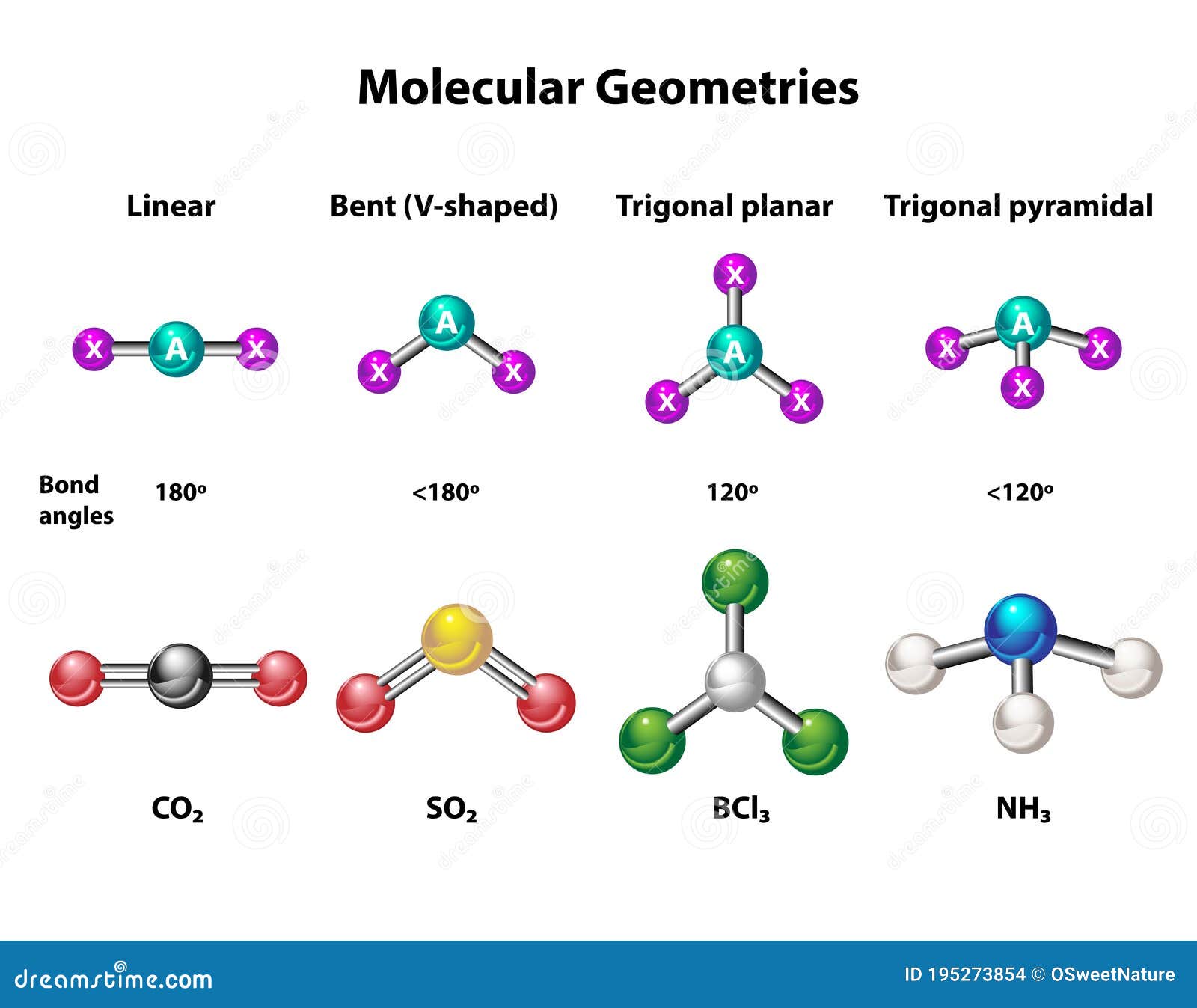
Molecular Geometry of AlCl3
The molecular geometry of AlCl3 is trigonal planar. This arrangement arises from the repulsion between electron pairs around the central aluminum atom. In a trigonal planar geometry, the bond angles between the chlorine atoms are approximately 120 degrees, ensuring the most stable configuration.
This geometry is crucial for understanding the compound's physical and chemical properties. The symmetric arrangement of atoms contributes to its nonpolar nature and influences its interactions with other substances.
Factors Influencing Molecular Geometry
- Electron pair repulsion theory (VSEPR): Determines the spatial arrangement of atoms based on minimizing repulsion between electron pairs.
- Number of bonding and lone pairs: AlCl3 has three bonding pairs and no lone pairs on the central atom.
Bonding in AlCl3
The bonding in AlCl3 involves covalent bonds between aluminum and chlorine atoms. Each chlorine atom shares one electron with aluminum, forming a stable compound. However, due to aluminum's incomplete octet, AlCl3 exhibits unique bonding characteristics that make it highly reactive.
This reactivity allows AlCl3 to act as a Lewis acid, accepting electron pairs from other molecules to complete its octet. This property is essential in various chemical reactions, particularly in Friedel-Crafts reactions.
Read also:Ask The 8 Ball Indra Your Ultimate Guide To Unlocking Lifes Answers
Types of Bonds in AlCl3
- Covalent bonds: Single bonds between aluminum and chlorine atoms.
- Dative bonds: Formed when AlCl3 accepts electron pairs from donor molecules.
Hybridization in AlCl3
In AlCl3, the central aluminum atom undergoes sp2 hybridization. This hybridization involves the mixing of one s orbital and two p orbitals to form three sp2 hybrid orbitals. These orbitals are used to form sigma bonds with the chlorine atoms, resulting in a trigonal planar geometry.
The remaining p orbital on aluminum is unhybridized and lies perpendicular to the plane of the molecule. This orbital plays a role in the compound's reactivity and ability to form complexes with other molecules.
Importance of Hybridization
- Determines the shape and geometry of the molecule.
- Influences the compound's reactivity and bonding capabilities.
Physical and Chemical Properties
AlCl3 exhibits several notable physical and chemical properties that make it a versatile compound in various applications. In its solid state, AlCl3 forms a lattice structure that can exist in two forms: anhydrous and hydrated. The anhydrous form is commonly used in industrial processes, while the hydrated form is less reactive.
Chemically, AlCl3 acts as a strong Lewis acid, making it a valuable catalyst in organic synthesis. Its ability to accept electron pairs allows it to participate in a wide range of reactions, including alkylation, acylation, and polymerization.
Key Properties
- Melting point: 192.6°C (anhydrous form).
- Boiling point: 182.7°C (sublimation).
- Solubility: Soluble in water, forming hydrochloric acid and aluminum hydroxide.
Applications of AlCl3
AlCl3 finds extensive use in various industries due to its unique properties. As a catalyst, it plays a crucial role in Friedel-Crafts reactions, which are essential for synthesizing aromatic compounds. Additionally, AlCl3 is used in the production of aluminum metal, water treatment, and as a drying agent in laboratories.
In the pharmaceutical industry, AlCl3 is utilized in the synthesis of drugs and intermediates. Its ability to form complexes with other molecules makes it valuable in the development of new materials and technologies.
Industrial Applications
- Catalyst in organic synthesis.
- Water purification and treatment.
- Production of aluminum metal.
Variations in AlCl3 Structure
Under certain conditions, AlCl3 can exist in different structural forms. In the solid state, it forms dimers (Al2Cl6) due to the formation of dative bonds between aluminum atoms. This dimeric structure is stabilized by the sharing of electron pairs and is prevalent in the anhydrous form.
In solution or vapor phase, AlCl3 can dissociate into individual monomers, depending on the solvent and temperature. These structural variations influence its reactivity and application in different environments.
Factors Affecting Structure
- Temperature and pressure conditions.
- Type of solvent and its polarity.
Synthesis of AlCl3
The synthesis of AlCl3 can be achieved through several methods, depending on the desired form and purity. The most common method involves the direct reaction of aluminum metal with chlorine gas at high temperatures. This reaction produces anhydrous AlCl3, which can be further processed for specific applications.
Alternatively, AlCl3 can be synthesized by reacting aluminum hydroxide with hydrochloric acid. This method is suitable for producing hydrated forms of the compound, which are less reactive but still useful in certain applications.
Synthesis Methods
- Direct reaction of aluminum and chlorine.
- Reaction of aluminum hydroxide with hydrochloric acid.
Safety Considerations
Handling AlCl3 requires proper safety precautions due to its reactive nature. It can cause severe irritation to the skin, eyes, and respiratory system. Ingestion or inhalation of AlCl3 can lead to serious health effects, including corrosion of tissues and organ damage.
When working with AlCl3, it is essential to use personal protective equipment (PPE) such as gloves, goggles, and a lab coat. Additionally, proper ventilation and containment measures should be implemented to minimize exposure risks.
Safety Tips
- Wear appropriate PPE when handling AlCl3.
- Ensure proper ventilation in the working area.
- Dispose of waste materials according to safety guidelines.
Conclusion
In conclusion, understanding the AlCl3 Lewis structure and molecular geometry provides valuable insights into its properties and applications. From its trigonal planar geometry to its role as a catalyst in organic synthesis, AlCl3 is a fascinating compound with diverse uses in various industries.
We encourage readers to explore further resources and experiments related to AlCl3. If you found this article helpful, please share it with others and consider leaving a comment with your thoughts or questions. Additionally, feel free to explore other articles on our website for more in-depth knowledge on chemistry and related topics.
Sources:
- https://pubchem.ncbi.nlm.nih.gov/compound/Aluminum-chloride
- https://www.sciencedirect.com/topics/chemistry/aluminum-chloride