The molecular shape of AlCl3 plays a crucial role in determining its chemical properties and reactivity. Aluminum chloride (AlCl3) is a well-known inorganic compound with a unique structure that influences its behavior in various chemical reactions. Understanding its molecular geometry is essential for students, researchers, and professionals in the field of chemistry.
AlCl3, also known as aluminum trichloride, is a versatile compound used in a wide range of industrial and laboratory applications. From catalysis to the production of aluminum metal, this compound has significant practical importance. However, to fully appreciate its utility, one must first understand its molecular structure and geometry.
In this comprehensive article, we will delve into the molecular shape of AlCl3, exploring its trigonal planar geometry, bond angles, electron configurations, and more. By the end of this article, you will have a clear understanding of why this molecule behaves the way it does and how its shape impacts its chemical properties.
Read also:Hikaru Nag The Rising Star In The World Of Chess
Table of Contents
- Introduction to Molecular Geometry
- Overview of Aluminum Chloride (AlCl3)
- Molecular Shape of AlCl3
- Trigonal Planar Geometry Explained
- Bond Angles in AlCl3
- Lewis Structure of AlCl3
- Hybridization in AlCl3
- Electronegativity and Polarity
- Industrial Applications of AlCl3
- Conclusion and Next Steps
Introduction to Molecular Geometry
Molecular geometry is a fundamental concept in chemistry that describes the three-dimensional arrangement of atoms in a molecule. This arrangement directly affects the molecule's physical and chemical properties, including its reactivity, polarity, and phase of matter. Understanding molecular geometry requires knowledge of electron configurations, bond angles, and the principles of VSEPR (Valence Shell Electron Pair Repulsion) theory.
Why Study Molecular Geometry?
The study of molecular geometry is essential for predicting how molecules will interact with one another. For instance, the shape of a molecule can determine whether it will form a strong covalent bond or participate in weak intermolecular forces. In the case of AlCl3, its molecular shape influences its behavior as a Lewis acid and its role in Friedel-Crafts reactions.
Overview of Aluminum Chloride (AlCl3)
Aluminum chloride (AlCl3) is an inorganic compound composed of aluminum and chlorine atoms. It exists in two forms: anhydrous (without water) and hydrated (with water). The anhydrous form is a white crystalline solid at room temperature, while the hydrated form is a deliquescent solid that absorbs moisture from the air.
Chemical Properties of AlCl3
- Formula: AlCl3
- Molar Mass: 133.34 g/mol
- Melting Point: 192.6°C (anhydrous)
- Boiling Point: 182.7°C (sublimation)
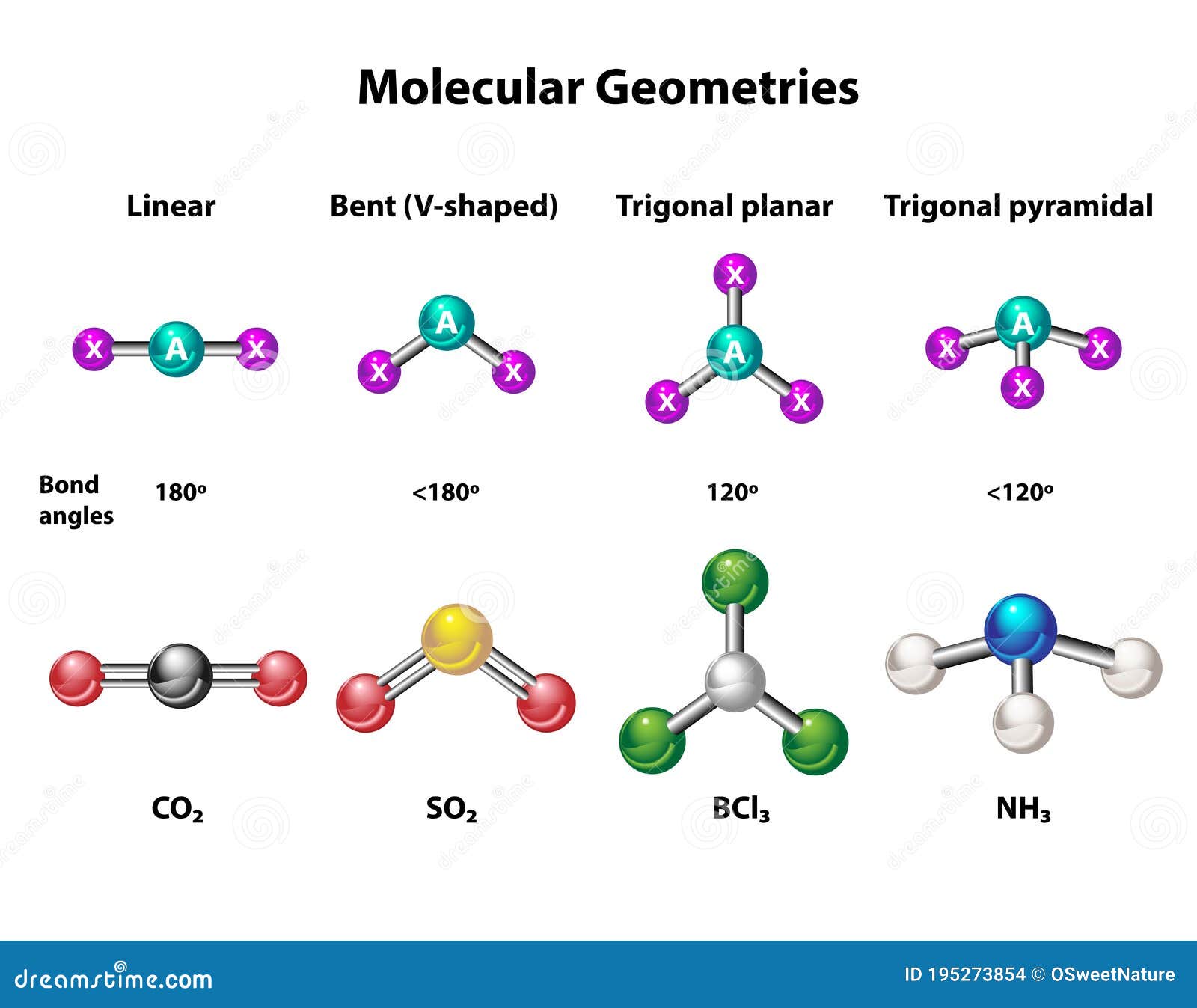
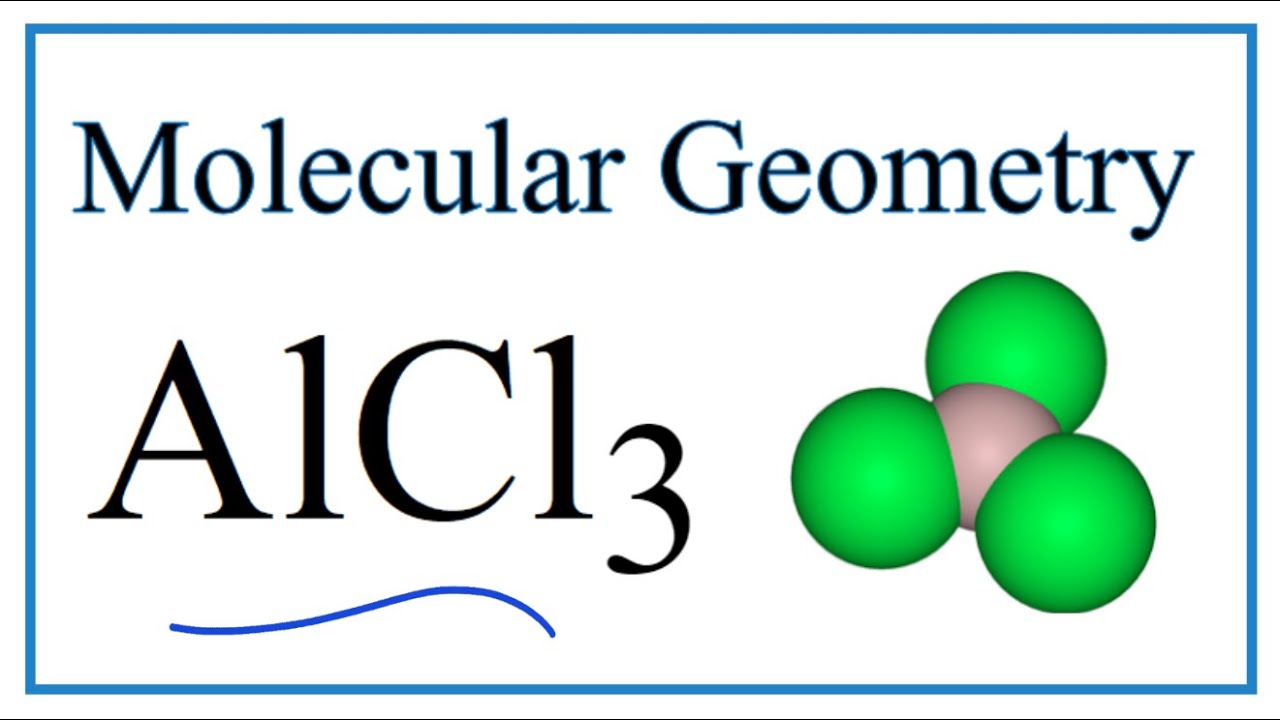
Molecular Shape of AlCl3
The molecular shape of AlCl3 is trigonal planar. This shape arises due to the arrangement of three chlorine atoms around a central aluminum atom. The aluminum atom forms three single covalent bonds with chlorine atoms, resulting in a flat, triangular geometry.
Factors Influencing Molecular Shape
The molecular shape of AlCl3 is determined by the number of bonding pairs and lone pairs of electrons around the central atom. In this case, aluminum has three bonding pairs and no lone pairs, leading to a trigonal planar geometry.
Trigonal Planar Geometry Explained
Trigonal planar geometry is a common molecular shape found in many compounds. It occurs when a central atom is bonded to three other atoms with no lone pairs of electrons. The bond angles in a trigonal planar molecule are typically 120°, ensuring minimal repulsion between electron pairs.
Read also:Sarah Marie Erome The Rise Of A Digital Phenomenon
In AlCl3, the three chlorine atoms are positioned at the vertices of an equilateral triangle, with the aluminum atom at the center. This arrangement maximizes the distance between electron pairs, minimizing repulsive forces and stabilizing the molecule.
Bond Angles in AlCl3
The bond angles in AlCl3 are precisely 120°. This value is consistent with the predictions of VSEPR theory, which states that electron pairs will arrange themselves to minimize repulsion. The trigonal planar geometry ensures that the chlorine atoms are equidistant from one another, maintaining stability in the molecule.
Importance of Bond Angles
Bond angles play a critical role in determining the reactivity and stability of a molecule. In the case of AlCl3, the 120° bond angles allow the molecule to maintain a balanced distribution of charge, making it an effective Lewis acid in various chemical reactions.
Lewis Structure of AlCl3
The Lewis structure of AlCl3 provides insight into its electron configuration and bonding. In this structure, the aluminum atom is represented at the center, surrounded by three chlorine atoms. Each chlorine atom shares one electron with aluminum, forming three single covalent bonds.
It is important to note that aluminum does not follow the octet rule in this compound. Instead, it has only six electrons in its outer shell, which is acceptable for elements in the third period of the periodic table.
Hybridization in AlCl3
The hybridization of AlCl3 is sp2. This means that the aluminum atom uses one s orbital and two p orbitals to form three hybridized orbitals. These orbitals are arranged in a trigonal planar geometry, allowing the aluminum atom to form three equivalent bonds with the chlorine atoms.
Significance of sp2 Hybridization
sp2 hybridization is responsible for the trigonal planar geometry of AlCl3. This type of hybridization ensures that the bonds between aluminum and chlorine are strong and stable, contributing to the molecule's overall reactivity.
Electronegativity and Polarity
Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. In AlCl3, chlorine is more electronegative than aluminum, resulting in polar covalent bonds. However, due to the symmetric arrangement of the chlorine atoms, the molecule as a whole is nonpolar.
Factors Affecting Polarity
The polarity of a molecule depends on both the electronegativity difference between atoms and the molecular geometry. In the case of AlCl3, the trigonal planar geometry cancels out the individual bond dipoles, making the molecule nonpolar.
Industrial Applications of AlCl3
Aluminum chloride (AlCl3) has numerous industrial applications due to its unique chemical properties. Some of the most significant uses include:
- Catalyst in Friedel-Crafts reactions
- Production of aluminum metal
- Water purification
- Manufacture of pharmaceuticals and pesticides
Its role as a Lewis acid makes it particularly valuable in organic synthesis, where it facilitates the formation of carbon-carbon bonds.
Environmental Considerations
Despite its utility, the use of AlCl3 in industrial processes raises environmental concerns. Proper disposal and handling are essential to minimize its impact on ecosystems and human health.
Conclusion and Next Steps
In conclusion, the molecular shape of AlCl3 is trigonal planar, a geometry that arises from the arrangement of three chlorine atoms around a central aluminum atom. This shape influences the compound's chemical properties, making it a versatile reagent in both industrial and laboratory settings.
We encourage readers to explore further resources on molecular geometry and its applications in chemistry. For those interested in learning more, consider consulting textbooks or academic journals on inorganic chemistry. Additionally, feel free to share this article with others who may find it informative. Your feedback and questions are always welcome in the comments section below!