Understanding the molecular geometry of AlCl3 is crucial for anyone interested in chemistry, particularly inorganic chemistry. This compound, aluminum trichloride, plays a significant role in various industrial applications, including the production of aluminum metal and as a catalyst in organic synthesis. Its structure and properties are directly influenced by its molecular geometry, making it an essential topic for both students and professionals in the field.
Aluminum trichloride (AlCl3) is a fascinating molecule that exhibits unique characteristics due to its molecular geometry. This compound is widely used in chemical industries and serves as a catalyst in several organic reactions. Understanding its molecular structure not only enhances our knowledge of inorganic chemistry but also aids in optimizing its applications in industrial processes.
This article delves into the molecular geometry of AlCl3, exploring its structure, properties, and applications. We will also discuss the factors influencing its geometry and how it affects the compound's behavior in different environments. By the end of this article, you will have a comprehensive understanding of AlCl3 and its significance in the world of chemistry.
Read also:Walmart Intercom Code Your Ultimate Guide To Understanding And Using It
Table of Contents
- Introduction to Molecular Geometry
- Structure of AlCl3
- Types of Molecular Geometry
- Factors Affecting Molecular Geometry
- Lewis Structure of AlCl3
- VSEPR Theory and AlCl3
- Hybridization in AlCl3
- Properties of AlCl3
- Applications of AlCl3
- Conclusion
Introduction to Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms in a molecule. It determines the shape of the molecule and influences its chemical properties and reactivity. Understanding molecular geometry is fundamental in chemistry, as it provides insights into how molecules interact with one another and their environment.
Importance of Molecular Geometry
The study of molecular geometry is crucial for predicting the behavior of molecules in various chemical reactions. It helps chemists understand the bond angles, bond lengths, and overall shape of molecules, which are essential for designing new materials and optimizing chemical processes.
For instance, the molecular geometry of AlCl3 affects its ability to act as a Lewis acid in organic reactions. Its trigonal planar shape enables it to accept electron pairs, making it an effective catalyst in Friedel-Crafts reactions.
Structure of AlCl3
Aluminum trichloride (AlCl3) consists of one aluminum atom bonded to three chlorine atoms. In its solid state, AlCl3 forms dimers (Al2Cl6) due to the formation of coordinate covalent bonds between aluminum atoms. However, in the gaseous state, it exists as monomeric AlCl3 with a trigonal planar geometry.
Monomeric vs. Dimeric Forms
In the solid state, AlCl3 exists as a dimer (Al2Cl6) due to the bridging of chlorine atoms between two aluminum atoms. This dimeric structure is stabilized by coordinate covalent bonds, where one chlorine atom donates its lone pair of electrons to the aluminum atom.
In the gaseous state, however, AlCl3 dissociates into its monomeric form, exhibiting a trigonal planar geometry. This change in structure is due to the reduced intermolecular forces in the gas phase.
Read also:Kimberly Violette Petty The Rising Star Shaping The Entertainment Industry
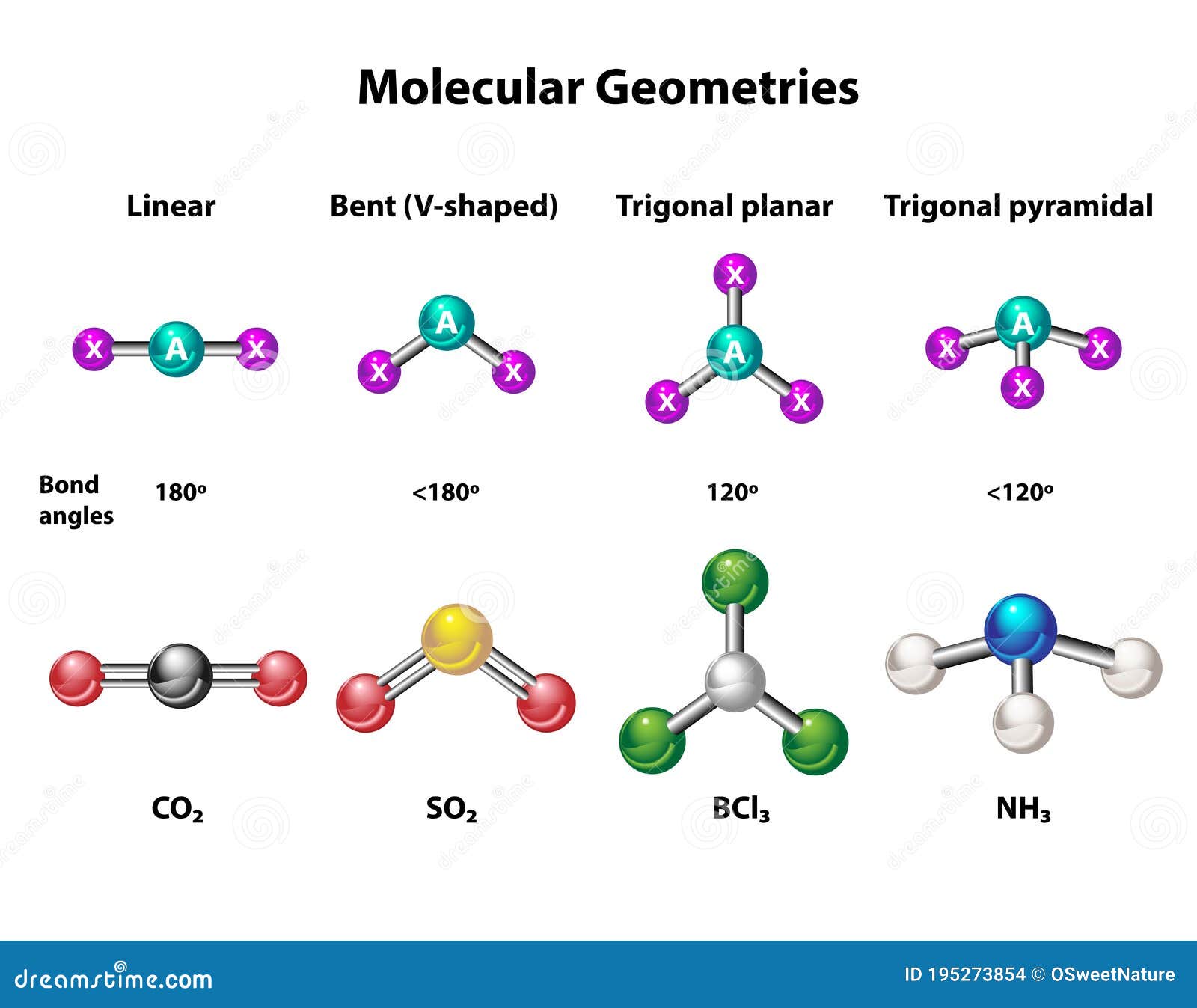
Types of Molecular Geometry
Molecular geometry can be classified into various types based on the number of electron pairs around the central atom. The most common geometries include linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral.
Trigonal Planar Geometry
AlCl3 adopts a trigonal planar geometry in its monomeric form. This geometry arises when a central atom is bonded to three surrounding atoms with no lone pairs of electrons. The bond angles in a trigonal planar molecule are approximately 120 degrees, as observed in AlCl3.
Besides AlCl3, other examples of molecules with trigonal planar geometry include BF3 (boron trifluoride) and SO3 (sulfur trioxide).
Factors Affecting Molecular Geometry
Several factors influence the molecular geometry of a compound, including the number of bonding pairs and lone pairs of electrons, electronegativity differences, and the size of the central atom.
Electron Pair Repulsion
The Valence Shell Electron Pair Repulsion (VSEPR) theory explains how electron pairs repel each other to achieve the most stable arrangement. In AlCl3, the three bonding pairs of electrons around the aluminum atom repel each other equally, resulting in a trigonal planar geometry.
Electronegativity differences between the central atom and surrounding atoms can also affect molecular geometry. In AlCl3, the difference in electronegativity between aluminum and chlorine influences the polarity of the bonds and the overall shape of the molecule.
Lewis Structure of AlCl3
The Lewis structure of AlCl3 represents the arrangement of valence electrons in the molecule. Aluminum, the central atom, contributes three valence electrons, while each chlorine atom contributes seven valence electrons. The total number of valence electrons in AlCl3 is 24.
Drawing the Lewis Structure
- Place aluminum at the center and chlorine atoms around it.
- Draw single bonds between aluminum and each chlorine atom.
- Complete the octets of the chlorine atoms by adding lone pairs of electrons.
- Aluminum does not satisfy the octet rule, as it has only six electrons in its valence shell.
This Lewis structure illustrates the electron arrangement in AlCl3 and helps predict its molecular geometry.
VSEPR Theory and AlCl3
The VSEPR theory provides a framework for predicting molecular geometry based on the repulsion between electron pairs. In AlCl3, the three bonding pairs of electrons around the aluminum atom repel each other equally, resulting in a trigonal planar geometry.
Applications of VSEPR Theory
VSEPR theory is widely used in chemistry to predict the shapes of molecules and their properties. By understanding the molecular geometry of compounds like AlCl3, chemists can design more efficient catalysts and optimize chemical reactions.
For example, the trigonal planar geometry of AlCl3 makes it an effective Lewis acid, capable of accepting electron pairs from nucleophiles in organic reactions.
Hybridization in AlCl3
Hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals that are used in bonding. In AlCl3, the aluminum atom undergoes sp2 hybridization, forming three equivalent sp2 hybrid orbitals that overlap with the p orbitals of the chlorine atoms to form sigma bonds.
Understanding Hybridization
Sp2 hybridization results in a trigonal planar geometry, as observed in AlCl3. The unhybridized p orbital on the aluminum atom is perpendicular to the plane of the molecule and does not participate in bonding.
Hybridization plays a crucial role in determining the shape and properties of molecules. In the case of AlCl3, sp2 hybridization contributes to its trigonal planar geometry and Lewis acid behavior.
Properties of AlCl3
Aluminum trichloride exhibits several unique properties due to its molecular geometry and chemical composition. Some of its key properties include:
- Low melting and boiling points due to its covalent nature.
- High reactivity as a Lewis acid in organic reactions.
- Hygroscopic behavior, meaning it readily absorbs moisture from the air.
- Corrosive nature, capable of reacting with metals and organic compounds.
Chemical Properties
AlCl3 acts as a Lewis acid by accepting electron pairs from nucleophiles. This property makes it an effective catalyst in Friedel-Crafts reactions, where it facilitates the formation of carbon-carbon bonds in aromatic compounds.
Additionally, AlCl3 can form complexes with various ligands, such as water and ammonia, due to its ability to accept electron pairs.
Applications of AlCl3
Aluminum trichloride finds extensive applications in various industries due to its unique properties. Some of its notable applications include:
- Catalyst in Friedel-Crafts reactions for the synthesis of aromatic compounds.
- Reagent in the production of aluminum metal through the Hall-Héroult process.
- Intermediate in the synthesis of other aluminum compounds, such as aluminum oxide and aluminum hydroxide.
- Component in the formulation of antiperspirants and deodorants.
Industrial Importance
The industrial significance of AlCl3 lies in its ability to enhance the efficiency of chemical processes. As a catalyst, it accelerates reactions without being consumed, making it a cost-effective choice for large-scale applications.
Furthermore, its role in the production of aluminum metal highlights its importance in the metallurgical industry, where it contributes to the development of lightweight materials for aerospace and automotive applications.
Conclusion
In conclusion, the molecular geometry of AlCl3 plays a vital role in determining its properties and applications. Its trigonal planar geometry arises from the sp2 hybridization of the aluminum atom and the repulsion between electron pairs. Understanding the structure and behavior of AlCl3 is essential for optimizing its use in chemical processes and industrial applications.
We encourage readers to explore further topics in molecular geometry and its applications. Feel free to leave a comment or share this article with others who may find it informative. For more insights into chemistry and related fields, explore our other articles on our website.